Your cart is currently empty!
Modeling First-Line Daratumumab Use for Newly Diagnosed, Transplant-Ineligible, Multiple Myeloma: A Cost-Effectiveness and Risk Analysis for Healthcare Payers
All your purchased models will be available under your account under “Dashboards”
Disease Area (Primary)
Multiple Myeloma
First Developed
04/21/2024
Last Developed
–
Software Used
R (e.g., heemod, BCEA, dampack, hesim)
Model Sponsor
Academic institution
Intervention
daratumumab_lenalidomide_and_dexamethasone
Model Validation Score
– %
Coming Soon In Phase II: You will be able to pay a fee to download the CADTH Tool for your model which includes subaggregated scores.
Results
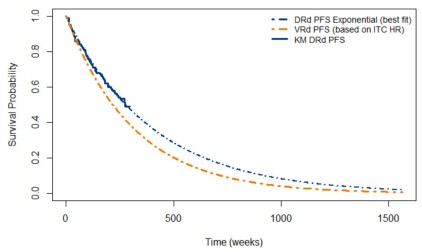
The incremental cost-effectiveness ratio (ICER) for daratumumab, lenalidomide, and dexamethasone (DRd) compared with bortezomib, lenalidomide, and dexamethasone (VRd) was US $90,364 per QALY gained. The results were sensitive to variations in survival for DRd, postprogression treatment costs, cost of hospice care, and hazard ratio for progression-free survival. The scenarios explored indicated that structural assumptions, such as the time horizon of the analysis, significantly influenced the results due to uncertainties arising from immature trial data and treatment efficacy over time. Among the various payer strategies compared, an upfront price discount for daratumumab emerged as the best approach with the lowest P-SUB at US $14,708.
Conclusion
What are the key conclusions or current applications of this model?
Source File(s)
Model:
Publication File:
Publication File 1:
Publication File 2:
Publication File 3:
Publication File 4:
No link submitted.
No link submitted.
No link submitted.
No link submitted.
Model Review
Only visible for the model owner
Summary
Validation Score
– %
Internal Comments
–